Brain Networks Interfacing with Reward Pathways:
Over thirty years of research has shown that the mesolimbic reward pathway plays a large role in reward related motivated behaviors, and that these circuits are modified by drugs of abuse. Recently, increased attention has focused on brain circuits that feed into this central reward pathway, since less is known about how these expanded networks regulate motivated behavior, and offers potentially avenues for treating disorders of motivation, like substance use disorders.
Lateral Hypothalamic Circuits
The Lateral Hypothalamus is a neuronally heterogeneous brain area normally studied in the context of brain homeostasis, energy balance, feeding, and alcohol drinking. Neurons in the Lateral Hypothalamus are connected to the mesolimbic reward pathway, and can directly influence multiple reward-seeking and reward-related behaviors.
Genetically targeting Lateral Hypothalamic Melanin-Concentrating Hormone Neurons
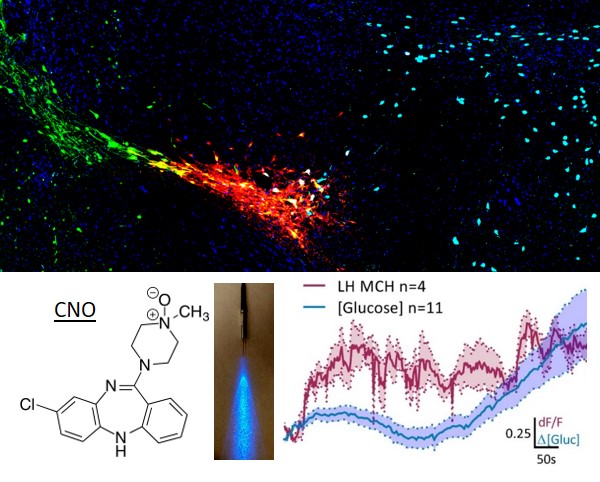 We use a variety of genetic targetting strategies to selectively deliver genes to specific neuronal subtypes. We use a combinatoral virus system, first developed by Caroline E. Bass at the University at Buffalo, SUNY, to drive expression of artificial transgenes in biochemically distinct neurons. Using this technology, we target neurons in the Lateral Hypothalamus like those that release Melanin-concentrating Hormone. Using this "toolbox approach", we can easily deliver transgenes such as channelrhodopsins and Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) that allow us to directly modulate the activity of a targeted neuron with compounds that have no behavioral effect on their own like clozapine-n-oxide (CNO), or with light, and transgenes that express Genetically Encoded Calcium Indicators (GECIs), which lets us measure the activity of these neurons by the influx of calcium associated with action potentials.
We use a variety of genetic targetting strategies to selectively deliver genes to specific neuronal subtypes. We use a combinatoral virus system, first developed by Caroline E. Bass at the University at Buffalo, SUNY, to drive expression of artificial transgenes in biochemically distinct neurons. Using this technology, we target neurons in the Lateral Hypothalamus like those that release Melanin-concentrating Hormone. Using this "toolbox approach", we can easily deliver transgenes such as channelrhodopsins and Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) that allow us to directly modulate the activity of a targeted neuron with compounds that have no behavioral effect on their own like clozapine-n-oxide (CNO), or with light, and transgenes that express Genetically Encoded Calcium Indicators (GECIs), which lets us measure the activity of these neurons by the influx of calcium associated with action potentials.
Glucose-Sensing Neurons in the Lateral Hypothalamus
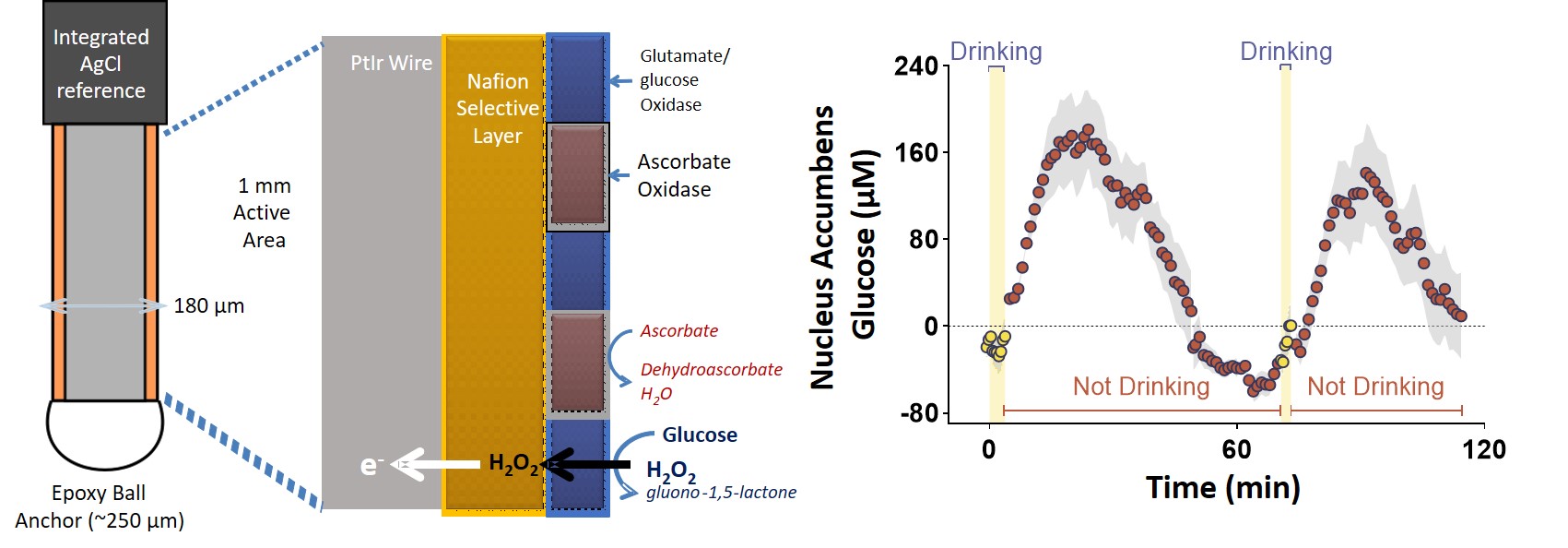 Many of the neurons in the Lateral Hypothalamus are glucose-sensitive, in that they change their firing patterns depending on the concentration of extracellular glucose outside the neuron. Glucose is one of the primary energy sources for the brain, and dynamically fluctuates depending on the glucose levels in the blood. In addition to fluctuations due to eating and drinking, brain glucose can also change due to energy demands associated with neuronal activity, and in response to drugs of abuse like cocaine and methamphetamine. Using in-vivo amperometry coupled to enzyme-linked biosensors, we measure the dynamic changes in lateral hypothalamic brain glucose concentrations at both the second-scale and minute-scale.
Many of the neurons in the Lateral Hypothalamus are glucose-sensitive, in that they change their firing patterns depending on the concentration of extracellular glucose outside the neuron. Glucose is one of the primary energy sources for the brain, and dynamically fluctuates depending on the glucose levels in the blood. In addition to fluctuations due to eating and drinking, brain glucose can also change due to energy demands associated with neuronal activity, and in response to drugs of abuse like cocaine and methamphetamine. Using in-vivo amperometry coupled to enzyme-linked biosensors, we measure the dynamic changes in lateral hypothalamic brain glucose concentrations at both the second-scale and minute-scale.
Neuronal Circuits Interfacing with Behavior
Using our cutting-edge basic neuroscience approaches, we can test the effects of modulating the activity of candidate target circuits on a battery of standard behaviors such as psychomotor sensitization, consummatory behavior, and operant responding.